CureLab Oncology's cancer therapy shows progress in clinical study
This weekend, CureLab Oncology is attending ESMO in Paris, where CureLab's science team will present the clinical benefits of its patented DNA drug. Dubbed Elenagen, the therapy is showing enormous promise in clinical trials focused on patients with platinum-resistant ovarian cancer (PROC), one of the deadliest cancers — and a disease that currently has no effective treatment modalities.
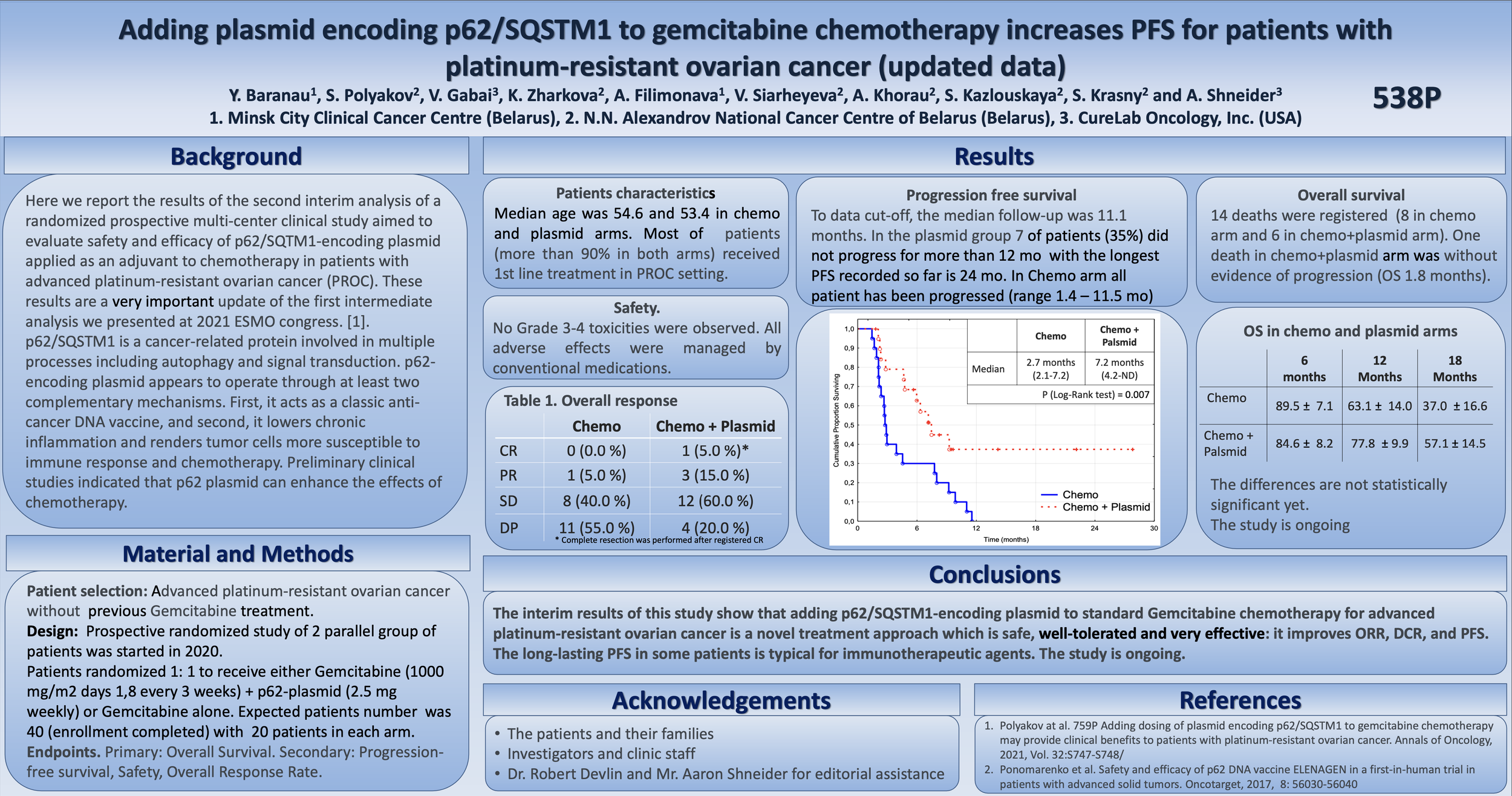
At ESMO, the team announced that the interim results for treatment of PROC patients with CureLab's p62 plasmid DNA are showing statistically significant changes in progression-free survival.
What's particularly noteworthy is that the clinical benefits currently being delivered for these terminally ill patients is already exceeding the benefits of new drugs recently approved by the FDA.
“Our presentation at ESMO 2022 is a great step forward compared to what we presented at ESMO 2021. Now, we are certain that Elenagen is increasing progression-free survival for platinum-resistant ovarian cancer patients in the trial.”
CLINICAL TRIAL INTERIM RESULTS
In the ex-US clinical trial, CureLab Oncology is assessing its experimental plasmid DNA therapeutic, Elenagen, for its ability to extend progression-free survival (PFS) in patients with PROC receiving standard chemotherapy, gemcitabine.
For all patients in the trial receiving the standard of care only, the disease progressed during the first year of observations, with 50% of patients progressing within 2.7 months.
In contrast, the disease did not progress at all for almost 40% of patients receiving the same gemcitabine in combination with weekly intramuscular injections of Elenagen.
In 50% of patients receiving standard of care only, the disease has progressed within 2.7 months, while the time to progression for 50% of patients in the “chemo plus Elenagen” group has increased to 7.2 months.
To date, no patient receiving Elenagen demonstrated any adverse event (AE) or significant adverse event (SAE) greater than grade 1, and all resolved quickly without impact.
So far, the longest PFS in the gemcitabine + Elenagen treatment group is 27 months (and continues to grow), while the disease has progressed in all patients in the gemcitabine-only group within less than 12 months.
“While our data on increasing progression-free survival are already statistically significant (P=0.01), it will take a little longer to reach a statistical significance on overall survival of the patients. However, so far, no single patient receiving gemcitabine alone has lived longer than 18 months, while over 50 percent of patients receiving gemcitabine and Elenagen live longer than that. Also of note is that no person who survived to live 18 months has died at this point in the study.”
ESMO 2022: interim results for treatment of platinum-resistant ovarian cancer with p62 plasmid DNA show statistically significant changes in progression-free survival